COVID-19 Antigen Rapid Test Cassette
- FOB Price:Get Latest Price >
- Min.Order:10000 Piece(s)
- Production Capacity:30000 pcs/day
- Payment Terms:T/T , D/P
- Favorite
Business Type:Manufacturer
Country/Region:China
Ddu Verified
HOT Rank


Wenzhou Astock Biotechnogy Co.,Ltd
We are professional supplier of Disposable virus sampling,Covid-19 igg/igm rapid test cassette,Nucleic acid detection kit,Colloidal gold test paper,Fluorescence quantitative PCR,Enzyme-labeled ELISA k
Business Type:Manufacturer
Country/Region:China
Ddu Verified
HOT Rank

Novel coronavirus antigen rapid detection kit
For professional use only.
For in vitro diagnosis only.
[expected usage]
The Novel Coronavirus Antigen Rapid Detection Kit is a lateral flow immunoassay method used by medical staff to qualitatively detect the novel coronavirus antigen in nasopharyngeal swabs and oropharyngeal swabs of patients suspected of being infected with novel coronavirus.
The test results are used to identify the new coronavirus nucleocapsid antigen. In the acute phase of infection, antigens are usually detectable in nasopharyngeal and oropharyngeal swabs. A positive result indicates the presence of viral antigens, but the clinical relevance of the patient's medical history and other diagnostic information is also necessary to determine the infection status. A positive result does not exclude bacterial infection or co-infection with other viruses. The detected pathogen may not be the exact cause of the disease.
Negative results cannot rule out new coronavirus infection, and therefore should not be used as the sole basis for treatment or patient management decisions (including infection control decisions). Negative results should be considered based on the patient’s recent exposure, medical history, and the presence of clinical signs and symptoms consistent with the new coronavirus pneumonia, and confirmed by nucleic acid analysis when necessary for patient management.
The new coronavirus antigen rapid detection kit is designed for use by trained clinical laboratory personnel who have been instructed and trained in specialized in vitro diagnostic procedures.
[Overview]
This new type of coronavirus belongs to the beta genus. Novel coronavirus pneumonia is an acute respiratory infectious disease. People are usually susceptible to infection. Currently, patients infected by the new coronavirus are the main source of infection. Asymptomatic infections can also be the source of infection. According to the current epidemiological survey, the incubation period is 1 to 14 days, most of which are 3 to 7 days. The main symptoms include fever, fatigue and dry cough. In a few cases, nasal congestion, runny nose, sore throat, myalgia and diarrhea occur.
[Inspection principle]
The new coronavirus antigen rapid detection kit is an immunoassay method based on the principle of double antibody sandwich technology. The new coronavirus antigen rapid detection kit is used to detect the nucleocapsid antigen of the new coronavirus in nasopharyngeal and oropharyngeal swabs of patients suspected of carrying the new coronavirus.
During the test, the sample migrates upward through the capillary effect. If present in the sample, the novel coronavirus antigen will bind to the antibody conjugate. Then, the immune complex is captured on the membrane by a pre-coated monoclonal antibody to the new coronavirus nucleocapsid protein, and a visible band will appear in the test line area, indicating that the result is positive. In the absence of the new coronavirus antigen, the test line area will not form a band, indicating that the result is negative.
In order to be used as a program control, the strip will always appear in the control line area, indicating that an appropriate volume of sample has been added and film wicking has occurred.
[Precautions]
·For in vitro diagnosis only.
·Suitable for healthcare professionals and point-of-care professionals.
·Do not use this product as the only basis for diagnosing or excluding new coronavirus infection or informing the status of new coronary pneumonia infection.
·Do not use after the expiration date.
·Before performing the test, please read all the information in this manual.
·Before use, the kit should be kept in a sealed bag.
· All samples should be considered as potentially dangerous and should be treated like the source of infection.
· Used kits should be discarded in accordance with federal, state and local regulations.
【composition】
The kit contains a membrane strip coated with monoclonal antibodies against the novel coronavirus nucleocapsid protein on the T test line and a staining pad containing colloidal gold and monoclonal antibodies against the novel coronavirus nucleocapsid protein.
The test quantity is printed on the label.
Provided equipment
·Kit ·Extraction tube·Drip tip·Extraction solution ·Workbench ·Instruction manual
Equipment required but not provided
• Timer
[Storage conditions and validity period]
· The original packaging should be stored at 4~30℃ (40-86F). The kit is stable during the expiry date indicated on the label.
· The kit should be used as soon as possible within 1 hour after the aluminum foil bag is opened. Prolonged exposure to hot and humid environments can cause product deterioration.
· Please refer to the label for batch and expiry date.
[Sample requirements]
Samples obtained early in the onset of symptoms will contain the highest viral titers; compared to RT-PCR analysis, samples obtained five days after the onset of symptoms are more likely to produce negative results. Insufficient sample collection, improper sample handling and/or transportation may produce false negative results; since sample quality is very important to produce accurate test results, it is strongly recommended that sample handling be trained.
Sample Collection
Nasopharyngeal swab sample
Insert a small pointed swab with a soft rod (wire or plastic) through the nostril parallel to the site (not upward) until resistance is encountered or the distance is equal to the distance from the ear to the patient’s nostril, indicating that it has been The depth of contact with the nasopharyngeal swab should be equal to the distance from the nostril to the outer opening of the ear. Wipe the swab gently and let it sit for a few seconds to absorb the secretions. Slowly remove the swab while rotating. The same swab can be used to collect samples from both sides, but if the microtip is filled with the liquid from the first collection, there is no need to collect samples from both sides. If a skewed septum or blockage makes it difficult to obtain a sample from one nostril, use the same swab to obtain a sample from the other nostril.
Oropharyngeal swab sample
Insert the swab into the back of the pharynx and tonsil area. Wipe in the tonsils and posterior oropharynx, avoiding contact with tongue, teeth and gums.
Sample Preparation
After collecting the swab sample, the swab can be stored in the extraction solution that comes with the kit. It can also be preserved by immersing the swab tip in a test tube containing 2 to 3 ml of virus preservation solution (or isotonic saline solution, tissue culture fluid or phosphate buffer).
Sample transportation and storage
The samples just collected should be processed as soon as possible, no later than one hour after the sample is collected. If not processed immediately, the collected samples should be stored at 2-8°C for no more than 24 hours. It can be stored for a long time at -70℃, but it is necessary to avoid repeated freeze-thaw cycles.
[Sample preparation]
1. Unscrew the extraction solution, add all the extraction solution to the extraction tube, and place it on the workbench.
2. Insert the swab sample into the extraction tube containing the extraction solution, and turn the swab at least 5 times while pressing the swab head on the bottom and sides of the extraction tube. Place the swab in the extraction tube for one minute.
3. Squeeze the side of the tube while taking out the swab to squeeze the solution from the swab. The extracted solution will be used as a test sample.
4. Insert the dripper firmly into the extraction tube.
(The picture is for reference only, please refer to the actual product for details.)
[Testing method]
Before the test, let the test equipment and the sample equilibrate to temperature (15-30℃ or 59.86P),
1. Remove the kit from the sealed bag.
2. Invert the sample extraction tube, put the sample extraction tube upright, transfer 3 drops (about 100μL) to the sample hole of the test box, and then start the timer. See the figure below.
3. Wait for the band to appear. Read the test results within 15 minutes. The read result is invalid after 20 minutes.
(The picture is for reference only, please refer to the actual product for details.)
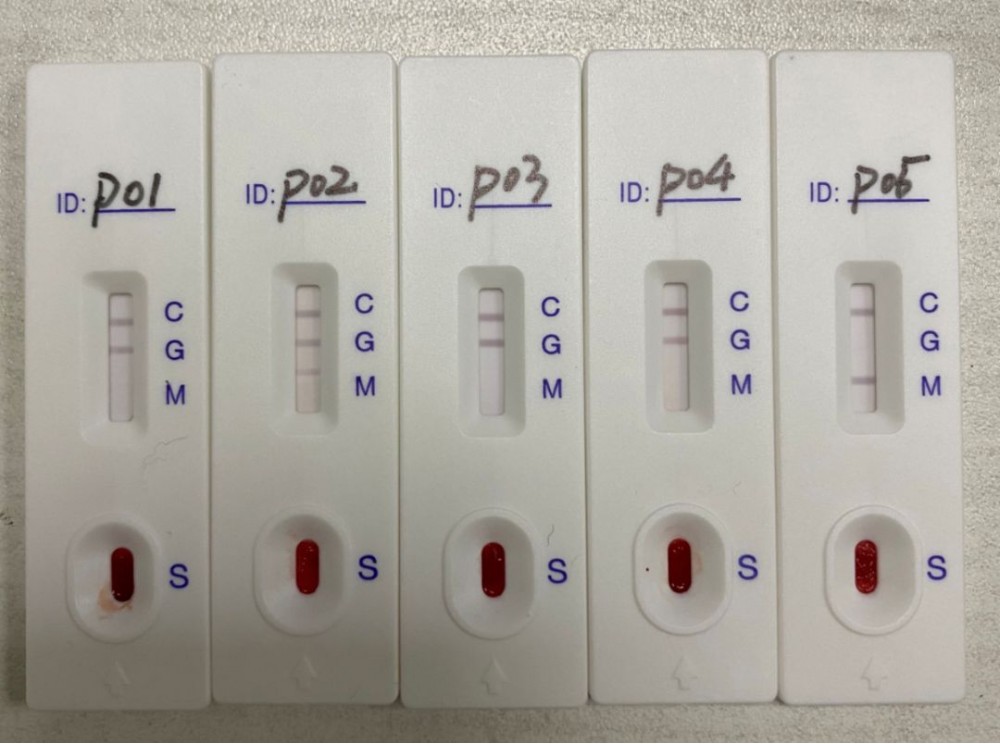
[Explanation of test results]
Positive negative invalid
Positive: Two bands appear. One strip should be in the control area (C) and the other strip should be in the test area (T). This indicates that it is positive for the presence of the new coronavirus nucleocapsid antigen. A positive result indicates the presence of viral antigens, but the clinical relevance of the patient's medical history and other diagnostic information is necessary to determine the infection status. A positive result does not exclude bacterial infection or co-infection with other viruses. The detected pathogen may not be the exact cause of the disease.
Negative: A band appears in the control area (C). No bands appear in the test area (T). The result is negative. A negative test result cannot rule out infection and should not be used as the sole basis for treatment or other patient management decisions (including infection control decisions), especially in patients who have clinical signs and symptoms consistent with new coronary pneumonia or have been in contact with the virus. It is recommended to confirm these results by nucleic acid testing when necessary for patient management.
Invalid: The control line cannot be displayed. Insufficient sample size or incorrect processing method is the most likely cause of control line failure. Repeat the test and repeat the test with a new test box. If the problem persists, please stop using the batch immediately and contact your local distributor.
[Quality Control]
A process control is included in the test. The purple-red band that appears in the control zone (C) is considered as internal process control. It ensures that the test requires a sufficient sample size, proper membrane phase shift and correct operating procedures.
This kit does not provide quality controls. However, it is recommended that laboratories use positive and negative controls for testing to confirm the testing procedures and properly verify the testing performance.
[Limitations of the test method]
· The new coronavirus antigen rapid test kit is limited to providing qualitative testing. The intensity of the detection is not necessarily related to the concentration of the sample antigen.
· A negative result does not rule out new coronavirus infection, so it should not be used as the sole basis for patient management decisions.
·Each doctor must combine the patient's medical history, physical examination results and other diagnostic procedures to interpret the results.
·If the number of antigens of the novel coronavirus in the sample is lower than the measured detection value, or the virus has a small amino acid mutation in the identified target epitope region, it may produce a negative result and pass the monoclonal used in the test antibody.
It is very important to collect specimens correctly, if you do not follow this procedure, you may get wrong results. Improper specimen collection, improper specimen storage, or repeated freezing and thawing of specimens can lead to inaccurate results.
【performance】
Detection limit (analysis sensitivity)
The test kit can detect the content of the novel coronavirus antigen not less than 5×102.67TCID50/mL.
Cross-reactivity (analysis accuracy)
We have studied the cross-reactivity with a certain concentration of the following virus or bacterial culture. The test results of the new coronavirus antigen rapid test kit are all negative:
Virus/bacteria concentration result
Influenza A virus (H1N1) 1×106PFU/mL -
Influenza A virus (H3N2) 1×106PFU/mL -
Influenza B virus (Yamagata) 1×106 PFU/mL -
Influenza B virus (Victoria) 1×106PFU/mL -
Adenovirus 1×106 PFU/mL -
Human interstitial pneumonia virus 1×106PFU/mL-
Parainfluenza virus 1×106 PFU/mL -
Respiratory syncytial virus 1×106PFU/mL -
Streptococcus pyogenes 1×107 CFU/mL -
Candida albicans 1×107 CFU/mL -
Mycoplasma pneumoniae 1×107 CFU/mL -
Chlamydia pneumoniae 1×107 CFU/mL -
Legionella pneumophila 1×107 CFU/mL -
Human Coronavirus 229E 1×106 PFU/mL -
Human Coronavirus OC43 1×106PFU/mL -
Human coronavirus NL63 1×106 PFU/mL -
Human Coronavirus HKU1 1×106 PFU/mL -
Clinical manifestations
In order to evaluate the clinical performance of the new coronavirus antigen rapid detection kit and compare it with RT-PCR, we collected 162 nasopharyngeal swabs from patients suspected of new coronary pneumonia.
The test data of nasopharyngeal swabs with the new coronavirus antigen rapid test reagent is summarized as follows:
RT-PCR sum
Novel coronavirus antigen positive negative
Novel coronavirus anti-positive 27 0 27
Original rapid test reagent negative 5 130 135
box
Total 32 130 162
Positive coincidence rate (PPA)=84.38% (27/32), (95%CI: 68.25%~93.14%)
Negative combination rate (NPA)=100% (130/130), (95%CI: 97.13%~100%)
[Manufacturing Enterprise] Wenzhou Astock Biotechnology Co., Ltd.
Registered address: No. 38 Dongfang Road, Wenzhou City, Zhejiang Province (Room 1208, Building 1, University Science Park Incubator)
Production address: No. 38 Dongfang Road, Wenzhou City, Zhejiang Province (Room 1208, Building 1, University Science Park Incubator)
Post Code: 325000